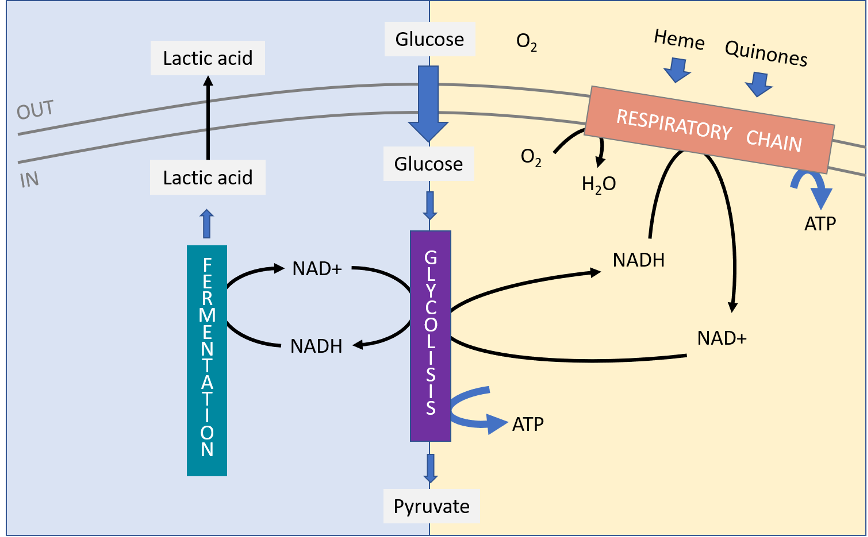
Science – Une nanomachine vivante : le moteur flagellaire et son contrôle électroprotonique
Une nanomachine vivante : le moteur flagellaire et son contrôle électroprotonique Le flagelle bactérien est bien plus qu’un simple appendice de propulsion : il constitue