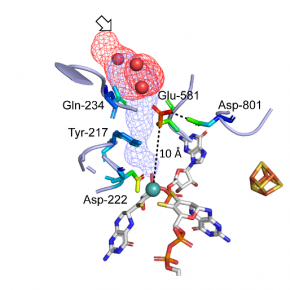
In this study, published in the journal ACS Catalysis, the group of Axel Magalon together with Guigliarelli’s team (BIP) and JP. Duneau (LISM) show that a group of conserved residues located at a long distance from the metal active site controls both substrate access and proton transfers, and thus strongly modulates the catalytic properties of nitrate reductase. These data provide a better understanding of the complex functioning of this molybdenum-based enzyme combining intramolecular transfers of substrate, protons and electrons in the catalytic process.
Read the ACS Catalysis article
Read the INSB article (French)