Science – Laurent Loiseau reçoit la médaille de cristal du CNRS 2025 ! 🏅
« La médaille de cristal distingue des femmes et des hommes, personnels d’appui à la recherche, qui par leur créativité, leur maîtrise technique et leur sens
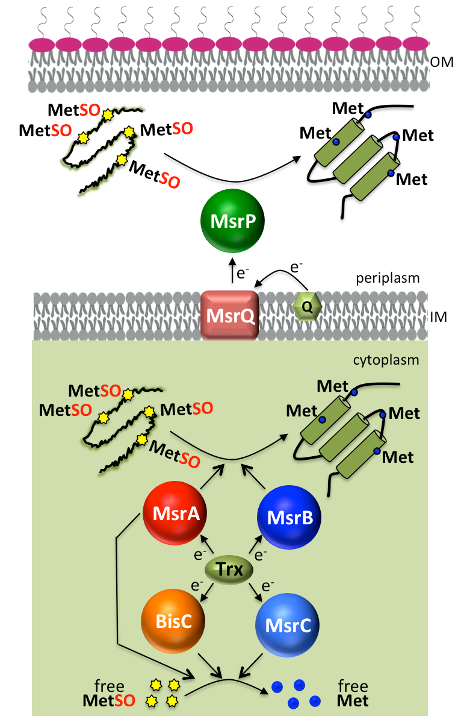
Our team aims at deciphering oxidative stress responses using Escherichia coli as a model bacterium and Salmonella enterica as pathogen bacterium. Proteins can be targeted and damaged by reactive oxygen species (ROS). Among all amino acids, methionine is the most sensitive residue to ROS, which can be converted to methionine sulfoxide (Met-SO). To rescue Met-SO containing proteins, living organisms synthesize methionine sulfoxide reductases (Msr). Enterobacteriaceae contains two cytosolic Msr (MsrA, MsrB) and one periplasmic Msr (MsrP). In addition, two other Msr (MsrC and BisC) are present in the cytoplasm where they can reduce only free Met-SO. The identification of protein targets and cellular processes under the surveillance of Msr is in progress. Our studies, both at the molecular and cellular levels, are orientated towards understanding of the contribution of each Msr in oxidative stress resistance, especially during bleach stress.
« La médaille de cristal distingue des femmes et des hommes, personnels d’appui à la recherche, qui par leur créativité, leur maîtrise technique et leur sens
Camille Andrieu is being awarded today the thesis prize of Aix Marseille University for her research conducted in the Ezraty team at Laboratoire de Chimie
« What is true for E. coli is true for the elephant« (J. Monod) Our team is working on the consequences of oxidative stress in bacteria.
Congratulations to Sara El Hajj for a super PhD defence, wishing you all the best for your future !
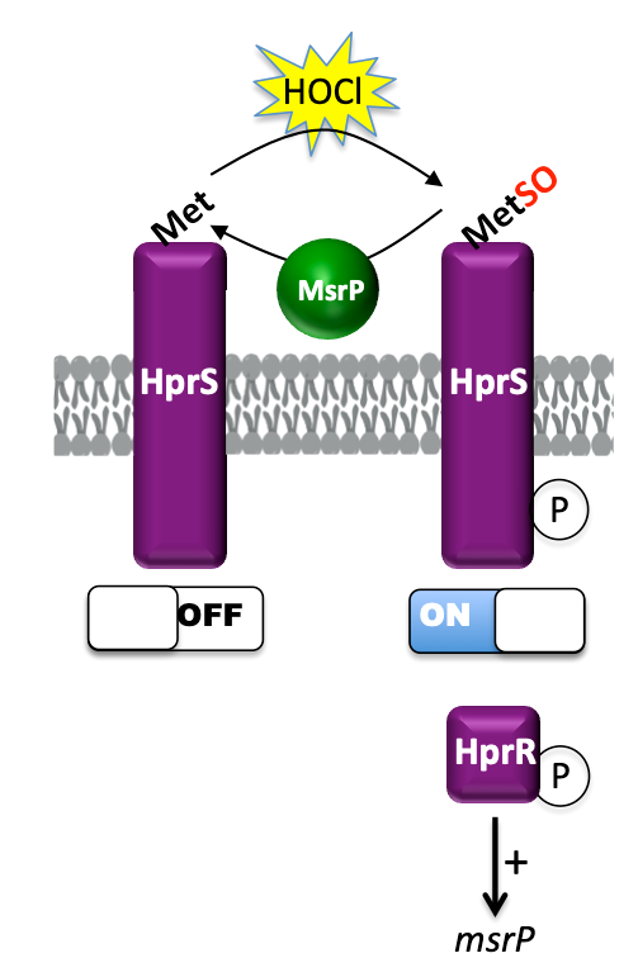
HprSR is a Reactive Chlorine Species-Sensing, Two-Component System in Escherichia coli : Read the article

Protein quality control is a vital cellular process. Bacteria are exposed to oxidative stress, both endogenous and exogenous, which eventually damage macromolecules, in particular proteins. Within proteins, sulfur-containing compounds such as methionine (Met) are targeted by both Reactive Oxygen Species (ROS) and Reactive Chlorine Species (RCS) with the latter being more efficient in converting Met into its oxidized methionine sulfoxide (Met-O) form. All living cells possess an intricate network of repair systems controlling the redox state of these residues that are highly prone to oxidation. Among them, methionine sulfoxide reductases (Msr) catalyze the reduction of Met-O into methionine residues.
Bacteria live in different environments and are subject to a wide variety of fluctuating conditions. Understanding how bacteria respond to oxidative stress at the molecular level is crucial in the fight against pathogens. HOCl is one of the most potent industrial and physiological microbiocidal oxidants. We study how bacteria sense HOCl by characterizing the HprSR two-component system in Escherichia coli.
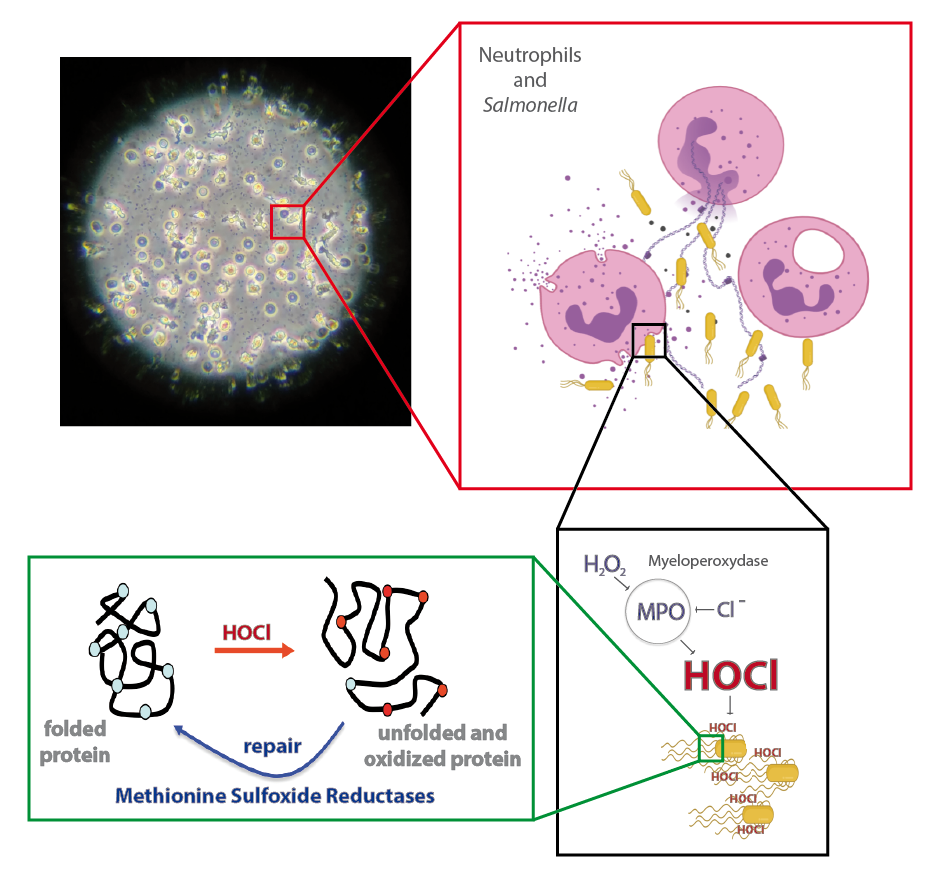
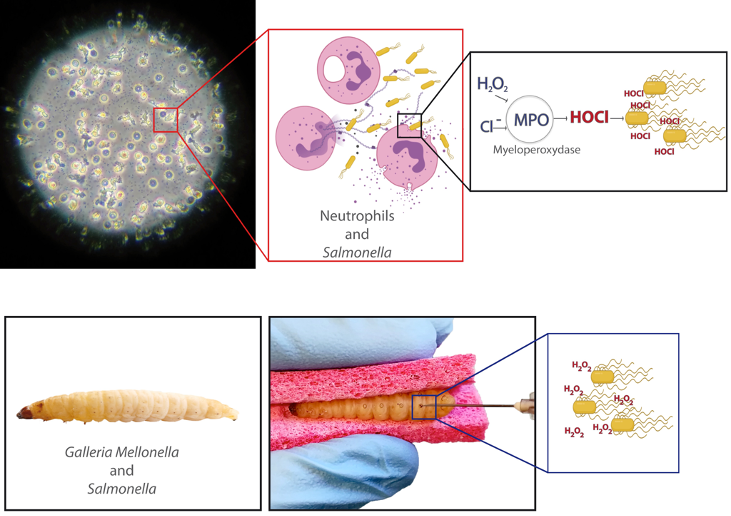
Immune cells such as hemocytes from Galleria mellonella larvae and neutrophils from vertebrate are among the first line of defense against invading pathogens. This antimicrobial activity relies on their ability to produce an oxidative burst. We aim at determining how Salmonella may respond to this stress.
Group Leader / Research director (DR-CNRS)
Scientific Project Leader / Researcher (CR-CNRS)
Professor (PR-AMU)
Research engineer (IR-CNRS)
Research engineer (CDD-CNRS)
Research engineer (CDD-CNRS)
PhD student (PhD-AMU)
PhD student (PhD-AMU)



